Quanterix Releases Operating Results for Third Quarter 2023
BILLERICA, Mass.- November 6, 2023 – Quanterix Corporation (NASDAQ: QTRX), a company fueling scientific discovery through ultrasensitive biomarker detection, today announced financial results for the three months ended September 30, 2023.
Third Quarter Financial Highlights
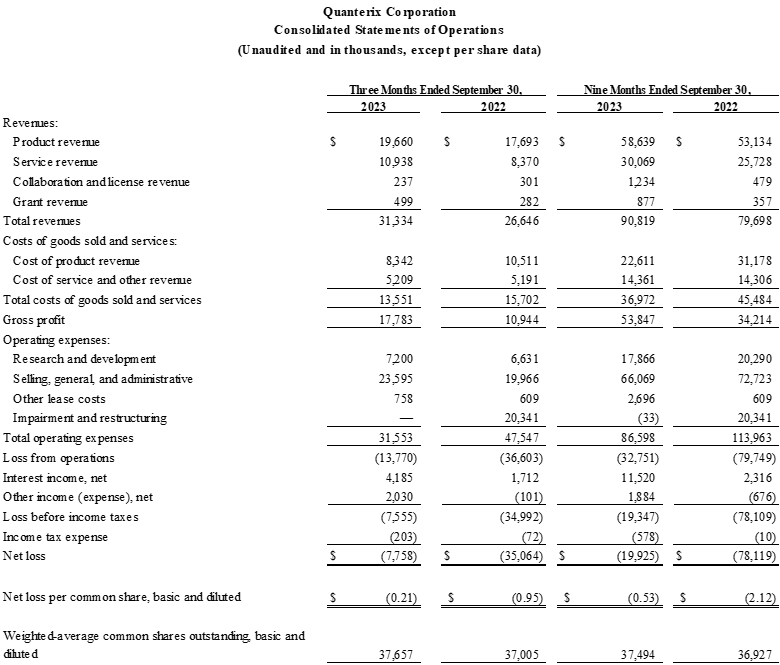
- Revenue was $31.3 million, an 18% increase from $26.6 million for the corresponding prior year period.
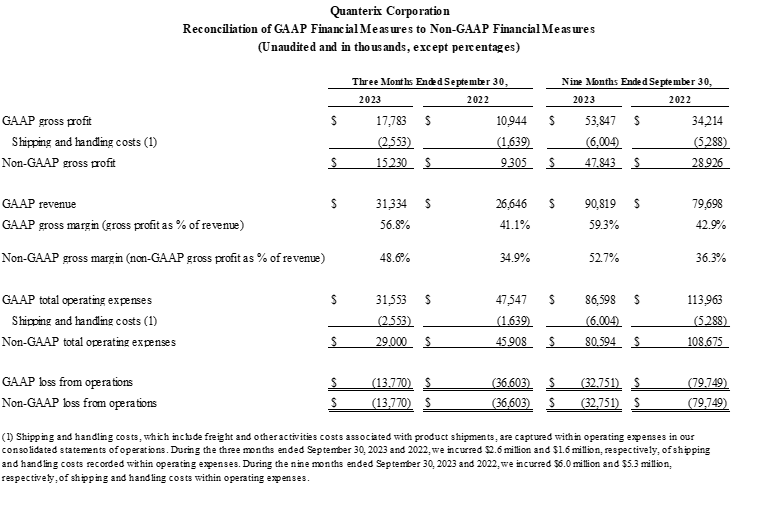
- GAAP gross margin was 56.8% as compared to 41.1% for the corresponding prior year period. Non-GAAP gross margin was 48.6% as compared to 34.9% for the corresponding prior year period.
- Net loss was $7.8 million as compared to $35.1 million for the corresponding prior year period.
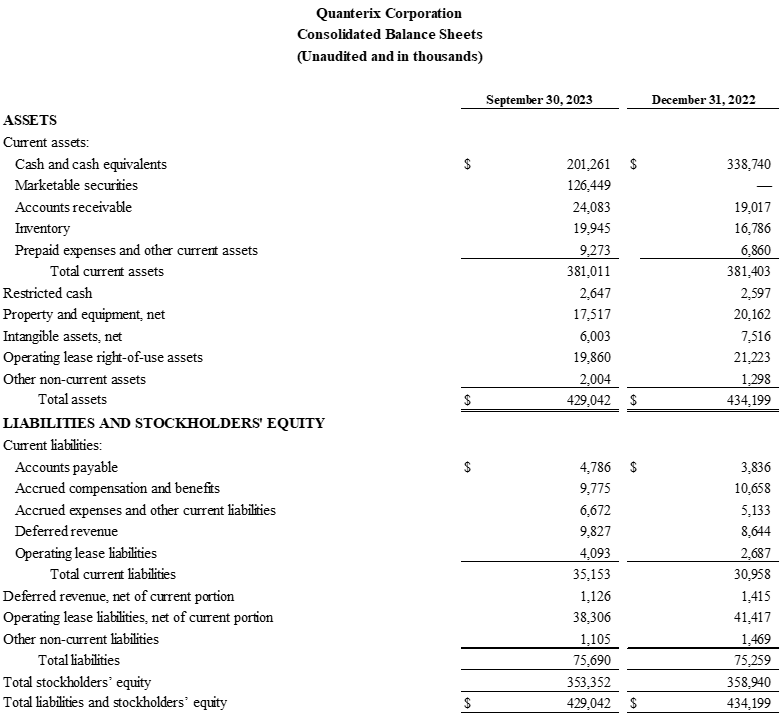
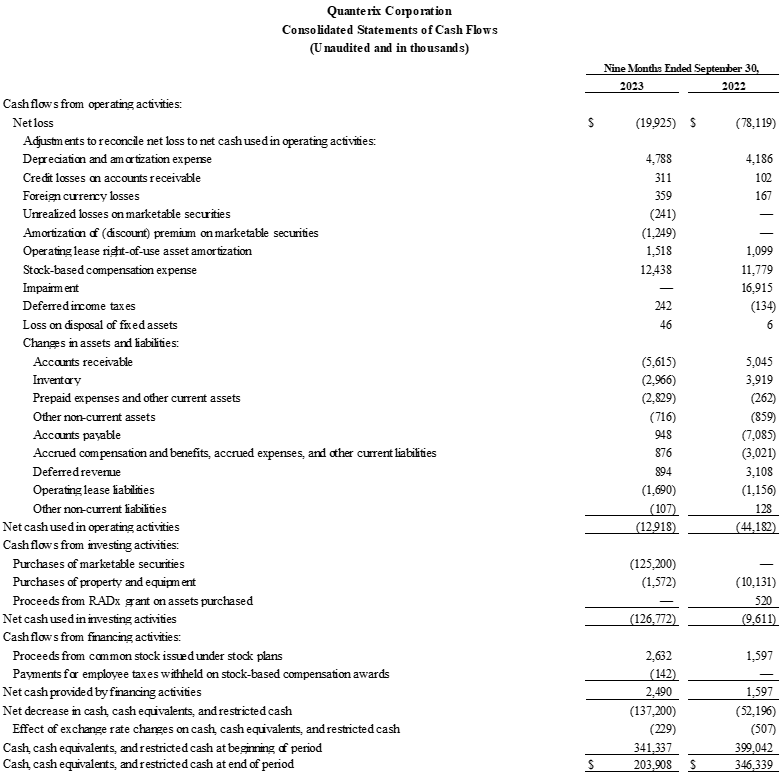
- Net cash use was approximately $1.9 million. Cash, cash equivalents, marketable securities, and restricted cash were $330.4 million as of September 30, 2023, as compared to $332.2 million as of June 30, 2023.
“We’re on track to achieve the six-quarter transformation plan we laid out by year end,” said Masoud Toloue, President and Chief Executive Officer of Quanterix. “This will not only yield highly scaled production lines but serve as the foundation for an accelerated innovation rate going into next year. Last month’s launch of our LucentAD p-Tau 217 blood-based test puts us in a leading position to address broad-based, non-invasive patient testing for Alzheimer’s disease. We expect a faster release pace of pioneering, high sensitivity Simoa products going into 2024.”
Operational and Business Highlights
- In October, the Company launched LucentAD p-Tau 217, a new blood-based biomarker laboratory developed test (LDT) using well-validated Johnson & Johnson Innovative Medicine (Janssen) antibodies to assist in the evaluation of patients suspected of having or developing Alzheimer’s disease. p-Tau 217 is the only blood-based biomarker recognized in the new draft NIA-AA Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease capable of meeting a stringent 90% accuracy criterion necessary to diagnose Alzheimer’s.
- In data presented by Eli Lilly at the CTAD conference, the Simoa® platform was used for analytical validation and initial clinical evaluation of Eli Lilly’s plasma p-Tau 217 immunoassay for a new blood-based diagnostic for Alzheimer’s disease. The study of over 1,000 patients from TRAILBLAZER-ALZ 2 demonstrated high positive and negative agreement to amyloid PET, with an AUC of 0.92 and the assay could prove to be a useful diagnostic test to identify the presence or absence of amyloid pathology.
Full Year Business Outlook
Management has increased full-year revenue expectations to be in the range of $118 to $120 million versus the prior range of $110 to $116 million. GAAP gross margin percentage is expected to be in the high 50’s, and non-GAAP gross margin percentage is expected to be approximately 50%. Both measures increased from prior guidance of low 50’s and high 40’s, respectively. The Company now anticipates 2023 cash usage in the range of $20 to $25 million, compared to prior guidance of $30 to $35 million.
For additional information on the non-GAAP financial measures included in this press release, please see “Use of Non-GAAP Financial Measures” and “Reconciliation of GAAP to Non-GAAP Financial Measures” below.
Conference Call
In conjunction with this announcement, the Company will host a conference call on November 7, 2023 at 8:30 a.m. E.T. Click here to pre-register for the conference call and obtain your dial-in number and passcode. Interested investors can also listen to the live webcast from the Event Details page in the Investors section of the Quanterix website at http://www.quanterix.com. An archived webcast replay will be available on the Company’s website for one year.
Financial Highlights
Use of Non-GAAP Financial Measures
To supplement our financial statements presented on a U.S. GAAP basis, we present non-GAAP gross profit, non-GAAP gross margin, non-GAAP total operating expenses, and non-GAAP loss from operations, which are calculated by including shipping and handling costs for product sales within cost of goods sold instead of within selling, general, and administrative expenses. Management uses these non-GAAP measures to evaluate our operating performance in a manner that allows for meaningful period-to-period comparison and analysis of trends in our business and our competitors. Management believes that presentation of these non-GAAP measures provides useful information to investors in assessing our operating performance within our industry and in order to allow comparability to the presentation of other companies in our industry where shipping and handling costs are included in cost of goods sold for products. Management also uses these non-GAAP measures as a factor in assessing our progress against our restructuring plan. The non-GAAP financial information presented here should be considered in conjunction with, and not as a substitute for, the financial information presented in accordance with U.S. GAAP.
Set forth below is a reconciliation of non-GAAP gross profit, non-GAAP gross margin, non-GAAP total operating expenses, and non-GAAP loss from operations to their most directly comparable GAAP financial measures.
Reconciliation of GAAP to Non-GAAP Financial Measures
About Quanterix
From discovery to diagnostics, Quanterix’s ultrasensitive biomarker detection is driving breakthroughs only made possible through its unparalleled sensitivity and flexibility. The Company’s Simoa technology has delivered the gold standard for earlier biomarker detection in blood, serum or plasma, with the ability to quantify proteins that are far lower than the Level of Quantification (LoQ) of conventional analog methods. Its industry-leading precision instruments, digital immunoassay technology and CLIA-certified Accelerator laboratory have supported research that advances disease understanding and management in neurology, oncology, immunology, cardiology and infectious disease. Quanterix has been a trusted partner of the scientific community for nearly two decades, powering research published in more than 2,200 peer-reviewed journals. Find additional information about the Billerica, Massachusetts-based company at https://www.quanterix.com or follow us on Twitter and LinkedIn.
Forward-Looking Statements
Quanterix’s current financial results, as discussed in this press release, are preliminary and unaudited, and subject to adjustment. This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements about Quanterix’s financial performance, including statements under the header “Full Year Business Outlook” set forth above, and are subject to a number of risks, uncertainties and assumptions. Forward-looking statements in this press release are based on Quanterix’s expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Factors that may cause Quanterix’s actual results to differ from those expressed or implied in the forward-looking statements in this press release include, but are not limited to, those described in the section titled “Part I, Item 1A, “Risk Factors” in Quanterix’ Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the U.S. Securities and Exchange Commission on March 6, 2023. Except as required by law, Quanterix assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
Contacts
Media:
PAN Communications
Maya Nimnicht
510-334-6273
quanterix@pancomm.com
Investor Relations:
Ed Joyce
(610) 306-9917
ir@quanterix.com