Quanterix Releases Operating Results for Second Quarter 2023
BILLERICA, Mass.- August 7, 2023 – Quanterix Corporation (NASDAQ: QTRX), a company fueling scientific discovery through ultrasensitive biomarker detection, today announced financial results for the three months ended June 30, 2023.
Second Quarter Financial Highlights
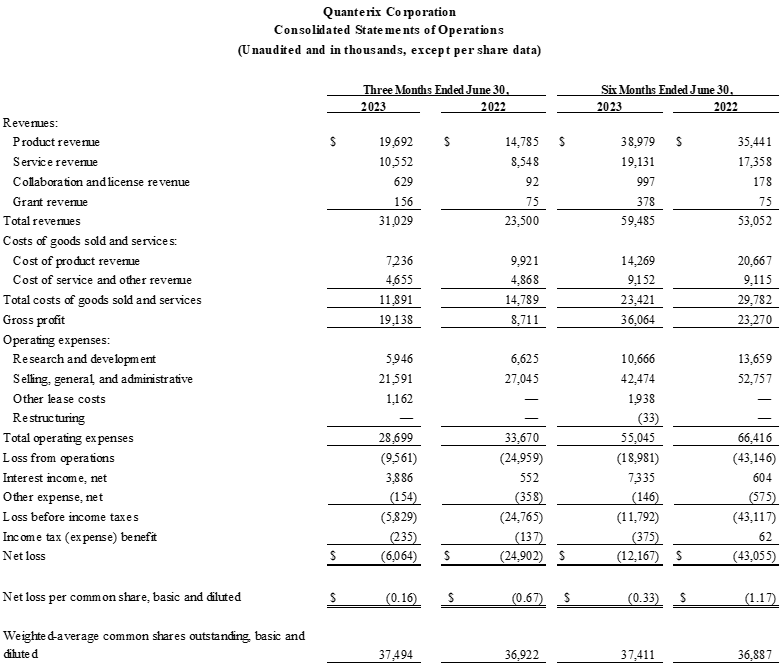
- Total revenue was $31.0 million versus prior year second quarter total revenue of $23.5 million, an increase of 32%. Second quarter revenue also increased 9% sequentially driven by increased Accelerator Lab and consumables revenue. Second quarter revenue includes a one-time benefit of approximately $1.5 million related to product and license agreements.
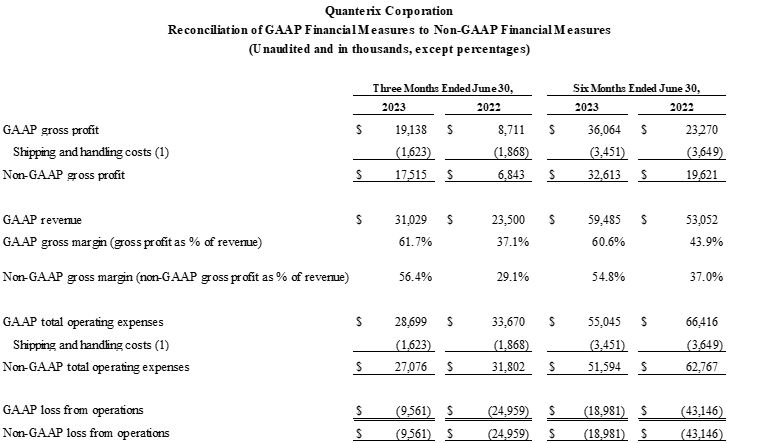
- GAAP gross margin was 61.7% compared to prior year second quarter GAAP gross margin of 37.1%. Non-GAAP gross margin was 56.4% compared to prior year second quarter non-GAAP gross margin of 29.1%.
- Net loss was $6.1 million compared to prior year second quarter net loss of $24.9 million.
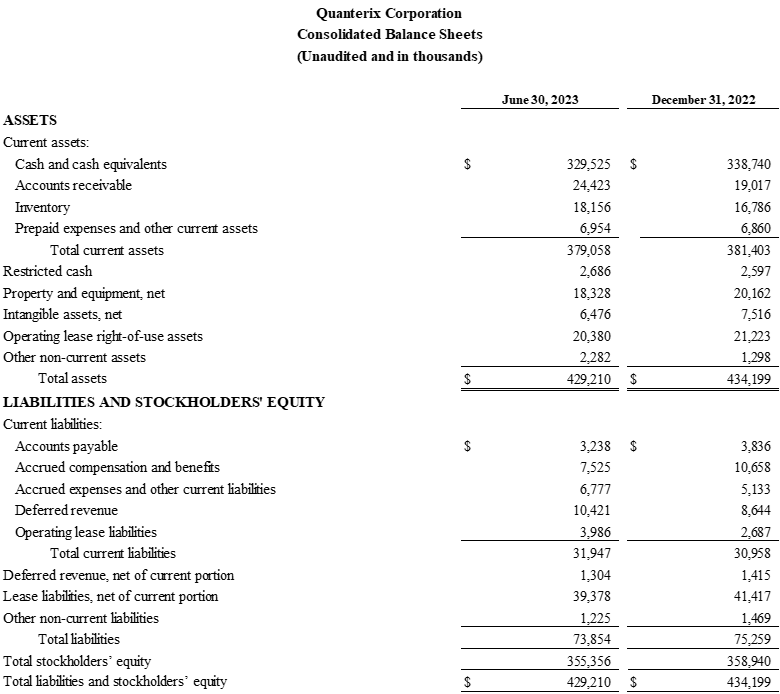
- Net cash flow in the quarter was approximately break-even. Cash and cash equivalents were $329.5 million as of June 30, 2023, as compared to $329.4 million as of March 31, 2023.
“Since launching our corporate transformation, we’re pleased with the accelerating progress as evidenced by both financial and business results,” said Masoud Toloue, President and Chief Executive Officer of Quanterix. “In under a year, our talented team has implemented fundamental change to business operations, with cash flow break-even being a key milestone, that said, we intend to continue to invest in scaling and supporting the important work performed on our Simoa® platform, that we believe will change the way we understand, test, and treat neurological diseases.”
Operational and Business Highlights
Accelerating our leadership position in blood-based biomarkers:
- In July, the Company launched LucentAD, a blood-based biomarker test to assist in the evaluation of patients experiencing cognitive symptoms consistent with the early signs of Alzheimer’s disease. This launch includes a new portal for patient and providers.
- Positive readout on Quanterix’s Bio-Hermes clinical study with the Global Alzheimer’s Platform Foundation confirmed high correlation between the LucentAD blood test and amyloid PET scans in early Alzheimer’s patients, a cohort most eligible for therapy. Results will be used to support Quanterix’s FDA filing.
- Quanterix’s Accelerator Lab will now provide Simoa assay testing for tau phosphorylation at residues 217 and 212 (p217+ tau), through a new research use only agreement with Janssen for its proprietary p217+ tau assays. Published studies have shown that the presence of phosphorylated tau in plasma is predictive of central amyloid and tau status and has the potential for diagnosing and staging Alzheimer’s disease.
- Robust reference dataset of serum NfL levels measured using Simoa technology across a wide spectrum of ages, from neonatal to 20-year-old adolescents, was published last week in Lancet Neurology, establishing a new standard with important implications on neuro-testing.
Full Year Business Outlook
With accelerating corporate transformation efforts, management has increased full-year revenue expectations to be in the range of $110 to $116 million, GAAP gross margin percentage to be in the low 50’s, and non-GAAP gross margin percentage to be in the high 40s. The Company also anticipates a lower 2023 cash burn, in the range of $30 to $35 million.
For additional information on the non-GAAP financial measures included in this press release, please see “Use of Non-GAAP Financial Measures” and “Reconciliation of GAAP to Non-GAAP Financial Measures” below.
Conference Call
In conjunction with this announcement, the Company will host a conference call on August 8, 2023 at 8:30 a.m. E.T. Click here to pre-register for the conference call and obtain your dial-in number and passcode.
Interested investors can also access the live webcast from the News & Events page within the Investors section of the Quanterix website at http://www.quanterix.com. An archived webcast replay will be available on the Company’s website for one year.
Financial Highlights
Use of Non-GAAP Financial Measures
To supplement our financial statements presented on a U.S. GAAP basis, we present non-GAAP gross profit, non-GAAP gross margin, non-GAAP total operating expenses, and non-GAAP loss from operations, which are calculated by including shipping and handling costs for product sales within cost of goods sold instead of within selling, general, and administrative expenses. Management uses these non-GAAP measures to evaluate our operating performance in a manner that allows for meaningful period-to-period comparison and analysis of trends in our business and our competitors. Management believes that presentation of these non-GAAP measures provides useful information to investors in assessing our operating performance within our industry and in order to allow comparability to the presentation of other companies in our industry where shipping and handling costs are included in cost of goods sold for products. Management also uses these non-GAAP measures as a factor in assessing our progress against the Restructuring Plan. The non-GAAP financial information presented here should be considered in conjunction with, and not as a substitute for, the financial information presented in accordance with U.S. GAAP.
Set forth below is a reconciliation of non-GAAP gross profit, non-GAAP gross margin, non-GAAP total operating expenses, and non-GAAP loss from operations to their most directly comparable GAAP financial measures.
Reconciliation of GAAP to Non-GAAP Financial Measures
About Quanterix
From discovery to diagnostics, Quanterix’s ultrasensitive biomarker detection is driving breakthroughs only made possible through its unparalleled sensitivity and flexibility. The Company’s Simoa ® technology has delivered the gold standard for earlier biomarker detection in blood, serum or plasma, with the ability to quantify proteins that are far lower than the Level of Quantification (LoQ) of conventional analog methods. Its industry-leading precision instruments, digital immunoassay technology and CLIA-certified Accelerator laboratory have supported research that advances disease understanding and management in neurology, oncology, immunology, cardiology and infectious disease. Quanterix has been a trusted partner of the scientific community for nearly two decades, powering research published in more than 2,200 peer-reviewed journals. Find additional information about the Billerica, Massachusetts-based company at https://www.quanterix.com or follow us on Twitter and LinkedIn.
Forward-Looking Statements
The Company’s current financial results, as discussed in this press release, are preliminary and unaudited, and subject to adjustment. This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements about Quanterix’ financial performance, including statements under the header “Full Year Business Outlook” set forth above, and are subject to a number of risks, uncertainties and assumptions. Forward-looking statements in this press release are based on Quanterix’ expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Factors that may cause Quanterix’ actual results to differ from those expressed or implied in the forward-looking statements in this press release include, but are not limited to, those described in “Part I, Item 1A, “Risk Factors” in Quanterix’ Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 6, 2023. Except as required by law, Quanterix assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
Contacts
Media:
PAN Communications
Maya Nimnicht
510-334-6273
quanterix@pancomm.com
Investor Relations:
Ed Joyce
(610) 306-9917
ir@quanterix.com